Synthesis, luminescence and electron–vibrational interactions of UV-excitable green phosphor α-Na2Ca4(PO4)2SiO4:Eu2+
Abstract
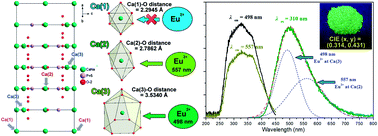
A series of UV-excitable α-Na2Ca4(PO4)2SiO4:Eu2+ green phosphors were synthesized by conventional solid-state reactions. The photoluminescence (PL) properties and concentration quenching of the as-prepared phosphors were investigated. All of the phosphors exhibited strong, broad absorption bands in the near-ultraviolet range and gave bright green emission upon excitation with 310 nm light. Eu2+ ions occupy 10-coordinated Ca(3) sites and 6-coordinated Ca(2) sites in α-Na2Ca4(PO4)2SiO4 and therefore generate two emission sub-bands at 497 and 557 nm. The electron–vibrational interaction (EVI) parameters, such as the Huang–Rhys factor, effective phonon energy and zero phonon line position, were calculated. With increasing Eu2+ concentration, the emission peak wavelength red-shifted from 510 to 530 nm, and the color hue can be tuned from green to yellowish green. The concentration quenching mechanism in α-Na2Ca4(PO4)2SiO4:Eu2+ consists of electric multipole–multipole interactions; these are most likely to be dipole–dipole and dipole–quadrupole interactions. These results indicate that α-NCPS:Eu2+ phosphors are promising candidates for application in NUV LEDs.


 Please wait while we load your content...
Please wait while we load your content...