Spectrometric and kinetics studies involving anionic chromogenic chemodosimeters based on silylated imines in acetonitrile or acetonitrile–water mixtures†
Abstract
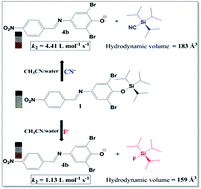
Three chromogenic anionic chemodosimeters (1–3) based on silylated imines were synthesized and characterized. Solutions of compound 1 in acetonitrile with 4.0% (v/v) of water are colorless, but with the addition of several anions only CN−, and to a lesser extent F−, changed the color of the solutions to orange. However, compounds 2 and 3 were selective toward F− in acetonitrile. The nucleophilic attack of F− or CN− on the silicon center of the chemodosimeters, through an SN2@Si mechanism, released colored phenolates as leaving groups. PGSE NMR data corroborated the mechanism postulated for the reaction. Kinetics studies were carried out, revealing that a higher second-order rate constant was obtained for the reaction of 1 with F−. The addition of water to the system reduces the nucleophilicity of F−, showing a slower second-order rate constant in relation to CN−, the latter anion being less hydrated and the more reactive species for the nucleophilic attack on the silicon center of 1.

 Please wait while we load your content...
Please wait while we load your content...