p-Bromoaryl- and ω-bromoalkyl-VA-PNBs: suitable starting materials for the functionalization of vinylic addition polynorbornenes via palladium-catalyzed cross-coupling reactions†
Abstract
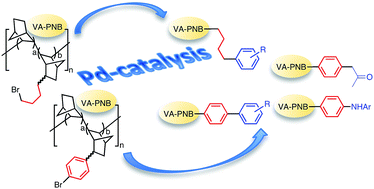
New vinylic addition polynorbornenes (VA-PNBs) with a variable amount of pendant 4-bromoaryl groups have been synthesized by homo- and copolymerization with norbornene of 2-(4-bromophenyl)-5-norbornene catalyzed by [Ni(C6F5)2(SbPh3)2]. These polymers can be transformed by Pd-catalyzed cross coupling reactions into VA-PNBs with pendant diaryl (Suzuki), alkenyl and ketoalkyl (Stille) and amino (Buchwald–Hartwig) groups. Although the cross-coupling reactions of alkylhalides are more difficult, (ω-bromobutyl)-VA-PNBs can also be functionalized by a Suzuki reaction with arylboronic acids. This results open up new ways of post-polymerization functionalization of VA-PNBs, quite attractive for their robust, thermally stable and transparent skeleton.


 Please wait while we load your content...
Please wait while we load your content...