One-step solvothermal synthesis of Al-promoted Fe3O4 magnetic catalysts for the selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water†
Abstract
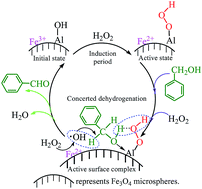
Aluminium (Al)-promoted Fe3O4 magnetic catalysts were prepared by in situ solvothermal synthesis in ethylene glycol and characterized by XRD, SEM, XPS, BET and VSM techniques. The Al promoter significantly enhanced the catalytic activity of Fe3O4 microspheres in the selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water (yield: 5.6% vs. 43.7% before and after the addition of 1.42 wt% of Al, respectively). The effects of Al source and content, catalyst loading, H2O2 dosage, reaction temperature and time on the catalytic performance of Al-promoted Fe3O4 were also investigated. The Al-promoted Fe3O4 catalysts were magnetically recoverable and reusable at least five times without loss of catalytic activity and selectivity after being rejuvenated with NaBH4 reduction. Theoretical and experimental investigations confirmed that the Al promoter introduced new Al active sites besides the primary iron (Fe) sites on the surface of Fe3O4 microspheres, facilitated the formation of Al–peroxo (Al–OOH) intermediates and reduced the apparent activation energy of the decomposition of H2O2 from 52.3 kJ mol−1 to 41.1 kJ mol−1. The synergistic effect between Fe and Al sites contributes to a much better catalytic activity of Al-promoted Fe3O4 as compared to pure Fe3O4, γ-Al2O3 or their physical mixtures as catalysts.

 Please wait while we load your content...
Please wait while we load your content...