Reaction intermediate/product-induced segregation in cobalt–copper as the catalyst for hydrogen generation from the hydrolysis of sodium borohydride
Abstract
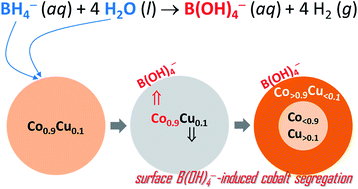
Cobalt is the most attractive catalyst for hydrogen generation from the hydrolysis of sodium borohydride, NaBH4, but its potential is further improved when it is combined with an inactive element like copper. Accordingly, several cobalt–copper catalysts (CoxCu1−x, with x as a mole ratio equal to 0, 0.1, 0.25, 0.5, 0.75, 0.9 or 1) were prepared. Under our conditions, Co0.9Cu0.1 shows the best performance, being able to complete H2 evolution in <4 min (vs. <7 min for Co). However, Co0.9Cu0.1 is not as stable as expected; after the first cycle, the catalytic activity in terms of the H2 generation rate halves, and then remains quite constant for additional cycles (up to five under our conditions). XPS measurements show that the surface composition of Co0.9Cu0.1 is subject to changes during hydrolysis; the anti-segregation of copper concomitantly takes place with the segregation of cobalt. This is explained through the occurrence of borate-induced segregation, favored due to the well-known strong affinity of cobalt for borate species. In other words, the catalytic activity of cobalt can be improved through combination with copper but, under our conditions, it cannot be stabilized. This is evidenced and discussed in detail herein.

 Please wait while we load your content...
Please wait while we load your content...