Characterization of Cd36_03230p, a putative vanillin dehydrogenase from Candida dubliniensis†
Abstract
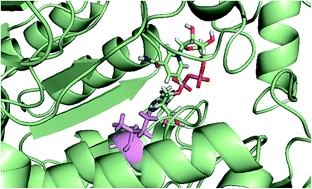
A coding sequence (CD36-03230) from the yeast Candida dubliniensis had been previously annotated as a vanillin dehydrogenase (VDH). The corresponding protein (CD36-03230p) was recombinantly expressed in Escherichia coli and analysed. The protein is most likely a tetramer in solution as judged by crosslinking and gel filtration experiments. CD36-03230p is an active aldehyde dehydrogenase favouring cyclic and aromatic substrates. Positive cooperativity and substrate inhibition were observed with some substrates. The redox cofactor NADP+ and substrates affected the thermal stability of the protein. Interestingly, the enzyme had no detectable activity with vanillin suggesting that the annotation is incorrect. It has been previously hypothesized that a methionine residue at a key position in the active site of yeast aldehyde dehydrogenases sterically hinders cyclic substrates and restricts specificity to aliphatic aldehydes. Molecular modeling of CD36-03230p demonstrates that it has an isoleucine residue (Ile-156) at this position, further strengthening this hypothesis.


 Please wait while we load your content...
Please wait while we load your content...