Effect of solvent ratio and counter ions on the morphology of copper nanoparticles and their catalytic application in β-enaminone synthesis†
Abstract
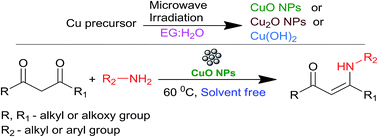
This work reports the synthesis of shape selective copper nanoparticles (NPs) using a microwave irradiation method, using diverse ratios of an ethylene glycol (EG)/water system. The solvent ratio of EG/water plays a pivotal role in the selective formation of CuO NPs. The alteration of the counter ion of the copper precursor leading to the selective synthesis of copper (CuO, Cu2O, Cu(OH)2) NPs has been observed. The synthesized copper NPs are well characterized using FEG-SEM, TEM, XRD, XPS, NH3-TPD and BET surface area. The prepared CuO NPs show high activity towards the synthesis of β-enaminones and β-enamino esters from 1,3 diketones and amines.


 Please wait while we load your content...
Please wait while we load your content...