Synthesis and biological evaluation of sapinofuranones A,B and 1,2,3-triazole-sapinofuranone hybrids as cytotoxic agents†
Abstract
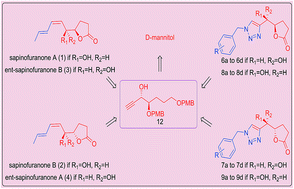
The total synthesis of sapinofuranones A,B and ent-sapinofuranones A,B and L-factor has been described. A series of novel 1,2,3-triazole-sapinofuranone hybrids were efficiently synthesized employing a click chemistry approach. These sapinofuranones and 1,2,3-triazole-sapinofuranone hybrids were further evaluated for their cytotoxic activity against four human cancer cell lines (A549, MDA-MB-231, DU145 and HepG2). Most of them revealed cytotoxic effects against cancer cells at the micromolar range. From a structure–activity relationship (SAR) perspective, it was noticed that the combination of triazole moiety to the lactone ring is playing modest role in exhibiting the cytotoxic effect. This is the first report on the synthesis and in vitro cytotoxic evaluation of 1,2,3-triazole-sapinofuranone hybrids.

 Please wait while we load your content...
Please wait while we load your content...