Nanosupramolecular assembly of amphiphilic guest mediated by cucurbituril for doxorubicin delivery†
Abstract
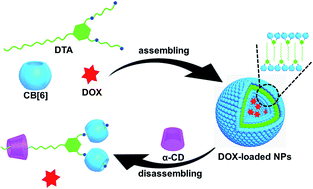
Nanosupramolecular amphiphilic assemblies have aroused significant attention in the biomaterials field and find potential applications in biomimetic synthesis, controlled/sustained drug release, and targeted imaging and diagnosis. In this work, a drug delivery system has been constructed by the host–guest complexation of cucurbit[6]uril (CB[6]) with a bifunctional guest compound (DTA) possessing one alkyl head and two ammonium tails. The assistance of CB[6] can dramatically decrease the critical aggregation concentration of this amphiphilic guest, thus leading to the formation of uniform and stable supramolecular nanoparticles (DTA·CB[6] NPs) at relatively lower concentration in water. Spectroscopic and microscopic experiments jointly demonstrate that the obtained NPs can not only encapsulate hydrophobic dye as molecular probe but also load hydrophilic drug (doxorubicin, DOX) in its internal microenvironment. In addition, when the hydrophobic region of DTA is included by α-cyclodextrin, the DTA·CB[6] NPs are completely dissipated and the encapsulated substrates can be released in a controlled manner. More interestingly, the DOX-loaded NPs exhibited better anticancer activity toward tumor cells, and therefore, the present study may provide a biocompatible platform in the construction of smart nanocarriers for on-demand drug delivery and release.

 Please wait while we load your content...
Please wait while we load your content...