An oligothiophene chromophore with a macrocyclic side chain: synthesis, morphology, charge transport, and photovoltaic performance†
Abstract
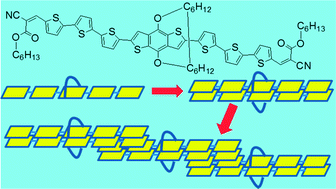
An oligothiophene chromophore RingBDT(T3A)2 has been synthesized, where BDT is benzo[1,2-b:4,5-b′]dithiophene, Ring is a 1,12-dodecylenedioxy cyclic side chain on the benzene of BDT, T3 is 2,2′:5′,2′′-terthiophene, and A is an electron acceptor. In single crystals, the immediate precursor of RingBDT(T3A)2 formed π-dimers and the ring prevented further π-stacking of the dimers. A differential scanning calorimetry study showed that BDT(T3A)2, the ringless analog with two 2-ethylhexyloxy side chains on BDT, crystallized quickly from its melt upon cooling, while crystallization of RingBDT(T3A)2 melt upon cooling was slow and incomplete. Interestingly, RingBDT(T3A)2 solid crystallized fast at ∼110 °C upon heating, but its thin films (200 nm) remained amorphous after annealing at 80 °C. Despite the amorphous nature, the hole mobility of RingBDT(T3A)2 films (1.52 × 10−3 cm2 V−1 s−1) was 144% higher than that of the highly crystalline BDT(T3A)2 films (200–80 nm). Solar cells were fabricated from blends of the chromophores and phenyl-C61-butyric acid methyl ester (PC60BM). Thermal annealing at 100 °C for 10 minutes enhanced chromophore π–π interaction, and improved device fill factor and efficiency for the RingBDT(T3A)2 blend solar cells, while retaining the amorphous nature of blend. In stark contrast, thermal annealing under the same conditions caused the efficiency of BDT(T3A)2 cell efficiency to drop by 82%. This study demonstrates the effectiveness of using a macrocyclic side chain as a strategy for developing amorphous molecular semiconducting materials with improved mobility and morphological stability.

 Please wait while we load your content...
Please wait while we load your content...