Rational design, synthesis and 2D-QSAR studies of antiproliferative tropane-based compounds†
Abstract
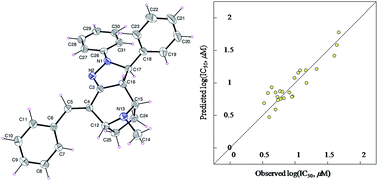
3,4-Diaryl-11-methyl-7-[(aryl)methylidene]-4,5,11-triazatricyclo[6.2.1.0*2,6*]undec-5-enes 14a–s were synthesized through reaction of 2,4-bis[(aryl)methylidene]-8-methyl-8-azabicyclo[3.2.1]octan-3-ones 12a–f with aryl hydrazines in the presence of catalytic amount of thiamine hydrochloride. Meanwhile, the 4-acetyl analogs 16a,b were obtained through reaction of 12b,e and hydrazine hydrate in acetic acid. Good support for the structure was received from single crystal X-ray studies of 14a. Some of the synthesized tropane containing-compounds showed promising antitumor properties during the in vitro MTT bio-assay against HepG2 (hepatocellular) and MCF7 (breast) human tumor carcinoma cell lines, with potency higher than that of doxorubicin (DNA intercalating agent, standard reference). Statistically significant 2D-QSAR model describes the antitumor properties against MCF7.


 Please wait while we load your content...
Please wait while we load your content...