Ammonia synthesis and by-product formation from H2O, H2 and N2 by dielectric barrier discharge combined with an Ru/Al2O3 catalyst
Abstract
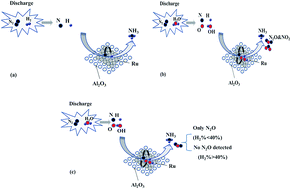
NH3 synthesis from H2O + N2, H2 + N2 or H2O + H2 + N2 by dielectric barrier discharge combined with an Ru/Al2O3 catalyst is studied at atmospheric pressure and room temperature. Additionally, the effects of reaction gas composition, energy density, and discharge frequency on NH3 yield and by-product formation are investigated. The results show that NH3 can be formed from reaction gases consisting of H2O and N2. The NH3 yield of the reaction gas containing H2 is much higher than that of the reaction gas consisting of H2O and N2. The presence of H2O has a promotion effect on NH3 synthesis from H2 and N2, especially for reaction gases with an H2 content less than 10%. The NH3 yield first increases and then decreases with an increase in the energy density. The maximum yield of 680 mg kW−1 h−1 occurs at the energy density of 1400 J L−1 and in the reaction gas of 0.14% H2O, 40% H2 and N2. The discharge frequency has a great effect on the NH3 yield, and maximum NH3 yield is obtained at the frequency of 13 kHz. Besides NH3, some by-products, such as N2O and NO2, are also formed with reaction gases containing H2O. However, their formation can be suppressed in the presence of H2, which is attributed to the reduction effect of H2 on the by-products.

 Please wait while we load your content...
Please wait while we load your content...