Solvent dependent isomerization of photochromic dithienylethenes: synthesis, photochromism, and self-assembly†
Abstract
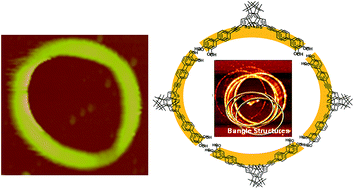
A series of photochromic dithienylethene incorporated with phenol (DTE1), catechol (DTE2), azophenol (DTE3) groups was synthesized and characterized by means of NMR spectroscopy, mass spectrometry and elemental analysis. The photophysical, photochromic and photoisomerization properties of molecules were studied using absorption and emission spectroscopies in different solvents. The cis–trans photoisomerizations of azophenols in DTE3 compete with photocyclization of dithienylethene, resulting in low photoconversion yields in DTE3. Thermal isomerization of cis-azophenols was found to be solvent-dependent – a fast thermal relaxation from cis- to trans-isomer in chloroform, and a slow process in THF. For example in chloroform, formation of a closed-ring dithienylethene with trans-azophenols (trans-DTE3closed) was observed upon irradiation with UV light (365 nm). On the other hand, closed-ring dithienylethene with cis-azophenols (cis-DTE3closed) was formed in THF under the same condition. After forming a complex with Fe3+ ions, DTE2 showed a red shift of 27 nm in the absorption maximum. Scanning electron microscopy (SEM) analysis revealed formation of circular nanostructures with diameters in the range of 300–600 nm from DTE2closed film. Factors such as solvent, photoisomerization, and hydrogen bonding toward the formation of such supramolecular nanostructures and morphologies are discussed.

 Please wait while we load your content...
Please wait while we load your content...