Selective production of methanol by the electrochemical reduction of CO2 on boron-doped diamond electrodes in aqueous ammonia solution†
Abstract
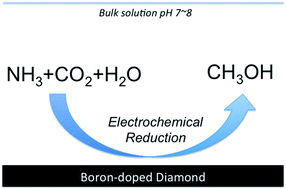
The electrochemical reduction of CO2 was investigated in an aqueous ammonia solution using boron-doped diamond electrodes. Methanol was mainly produced by reduction at a potential of −1.3 V (vs. Ag/AgCl) with a faradaic efficiency as high as 24.3%. Also, even in an aqueous ammonium bicarbonate solution (pH 7.9) without CO2 bubbling, methanol was produced, while no methanol production was observed at higher (10.6) and lower pH (3.38). These observations suggest that the selectivity for methanol production in aqueous ammonia solutions is due to the electrochemical reduction of bicarbonate ions which are formed by the reaction between ammonia and CO2. Moreover, we present the important role of ammonia as an electrolyte for the selective production of methanol by electrochemical reduction of CO2.

 Please wait while we load your content...
Please wait while we load your content...