Higher-order structure formation of a poly(3-hydroxybutyrate) film during solvent evaporation
Abstract
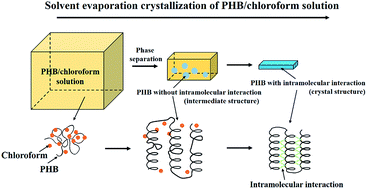
Solvent evaporation crystallization (SEC) of poly(3-hydroxybutyrate) (PHB) in a PHB/chloroform solution was investigated by time-resolved attenuated total reflection Fourier-transform infrared (ATR-FTIR) spectroscopy and grazing incidence wide angle X-ray diffraction (GI-WAXD). The ATR-FTIR investigation reveals that the PHB/chloroform solution was in a homogeneous state at first, and with the evaporation of chloroform, the separated PHB from the chloroform solvent was in the mixture of intermediate and amorphous states, but no crystal structure formed due to the presence of chloroform. Subsequently, further evaporation induced a transition from intermediate to crystal structure and the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–C intramolecular interactions within the latter. As the crystal structure developed, the intra-molecular interaction changed from weak to strong due to the reduced intra-molecular distance within the lamella structure. The results of the GI-WAXD investigation suggest the presence of two kinds of intermediate structures with different order (less ordered and highly ordered). During SEC, the intermediate structures formed first, subsequently transforming into a crystal structure.
O⋯H–C intramolecular interactions within the latter. As the crystal structure developed, the intra-molecular interaction changed from weak to strong due to the reduced intra-molecular distance within the lamella structure. The results of the GI-WAXD investigation suggest the presence of two kinds of intermediate structures with different order (less ordered and highly ordered). During SEC, the intermediate structures formed first, subsequently transforming into a crystal structure.

 Please wait while we load your content...
Please wait while we load your content...