Novel triphenylamine based rhodamine derivatives: synthesis, characterization, photophysical properties and viscosity sensitivity†
Abstract
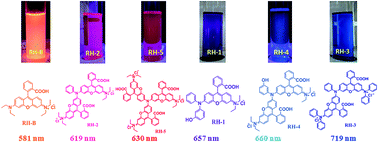
Five novel triphenylamine based deep red to NIR emitting rhodamine derivatives were synthesized and characterized by 1H and 13C NMR spectroscopy and elemental analysis. The photophysical properties of all these derivatives were studied in their spirocyclic as well as open form. Hydroxyl substituted derivatives were found to show red shifted emissions as compared to the plain rhodamine derivatives, while triphenylamine substituted derivatives showed larger Stokes shift and emission in the NIR region. All these newly synthesized rhodamine derivatives show comparatively larger Stokes shift (44–135 nm) than the commercially available Rhodamine B and Rhodamine 101. In their open form they are found to exhibit different emission color from pink (619 nm) to dark blue (719 nm) in day as well as UV-light. We also studied the interconversion of dye RH-2 from its spirocyclic to open form with the addition of acid (TFA in toluene). They are studied for their viscosity sensitivity and found to show very high fluorescence enhancement in polar viscous media such as ethanol–glycerol in their open form.


 Please wait while we load your content...
Please wait while we load your content...