Band and bonding characteristics of N2+ ion-doped graphene
Abstract
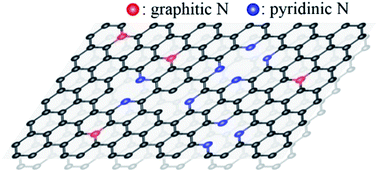
We report that the doping of energetic nitrogen cations (N2+) on graphene effectively controls the local N–C bonding structures and the π-band of graphene critically depending on ion energy Ek (100 eV ≤ Ek ≤ 500 eV) by using a combined study of photoemission spectroscopy and density functional theory (DFT) calculations. With increasing Ek, we find a phase transformation of the N–C bonding structures from a graphitic phase where nitrogen substitutes carbon to a pyridinic phase where nitrogen loses one of its bonding arms, with a critical energy Eck = 100 eV that separates the two phases. The N2+-induced changes in the π-band with varying Ek indicate an n-doping effect in the graphitic phase for Ek < Eck but a p-doping effect for the pyridinic graphene for Ek > Eck. We further show that one may control the electron charge density of graphene by two orders of magnitude by varying Ek of N2+ ions within the energy range adopted. Our DFT-based band calculations reproduce the distinct doping effects observed in the π-band of the N2+-doped graphene and provide an orbital origin of the different doping types. We thus demonstrate that the doping type and electron number density in the N2+ ion-doped SLG can be artificially fine-controlled by adjusting the kinetic energy of incoming N2+ ions.

 Please wait while we load your content...
Please wait while we load your content...