Enhanced drug release by selective cleavage of cross-links in a double-cross-linked hydrogel†
Abstract
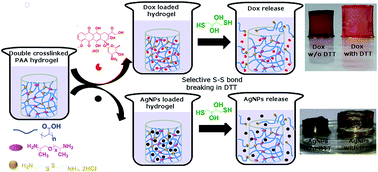
In the present paper, we report on the synthesis and characterization of redox sensitive double-cross-linked poly(acrylic acid) hydrogels using two different cross-linking agents, Jeffamine® and cystamine. The amount of two cross-linking agents was varied in order to synthesize hydrogels with different mechanical strengths. Jeffamine provides mechanical stability to the hydrogels while cystamine incorporates redox sensitivity. The stress values at the break point of the mono- and double-cross-linked hydrogels were determined from stress–strain plots. The disulphide bonds (S–S) in the cystamine were cleaved selectively in the presence of dithiothreitol, which increased the degree of hydrogel swelling. This phenomenon of in situ breaking of one cross-linking and increasing the swelling ratio could be used in swelling-controlled drug delivery systems. The implication of selective breaking of cross-links on the swelling-controlled release of the anticancer drug doxorubicin was demonstrated. We also successfully prepared Ag nanoparticles in the dual cross-linked hydrogels in order to incorporate antibacterial properties and studied their release by selective cleavage of cystamine bonds. These double-cross-linked hydrogels show great promise in drug delivery and tissue engineering applications.

 Please wait while we load your content...
Please wait while we load your content...