Synthesis of dimeric analogs of adenophostin A that potently evoke Ca2+ release through IP3 receptors†
Abstract
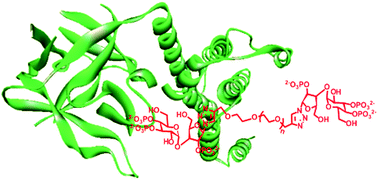
Inositol 1,4,5-trisphosphate receptors (IP3Rs) are tetrameric intracellular channels through which many extracellular stimuli initiate the Ca2+ signals that regulate diverse cellular responses. There is considerable interest in developing novel ligands of IP3R. Adenophostin A (AdA) is a potent agonist of IP3R and since some dimeric analogs of IP3R ligands are more potent than the corresponding monomer; we considered whether dimeric AdA analogs might provide agonists with increased potency. We previously synthesized traizolophostin, in which a simple triazole replaced the adenine of AdA, and showed it to be equipotent to AdA. Here, we used click chemistry to synthesize four homodimeric analogs of triazolophostin, connected by oligoethylene glycol chains of different lengths. We evaluated the potency of these analogs to release Ca2+ through type 1 IP3R and established that the newly synthesized dimers are equipotent to AdA and triazolophostin.

 Please wait while we load your content...
Please wait while we load your content...