The persistent energy transfer of Eu2+ and Dy3+ and luminescence properties of a new cyan afterglow phosphor α-Ca3(PO4)2:Eu2+,Dy3+
Abstract
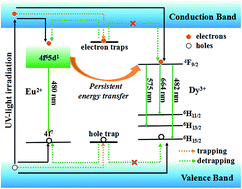
A new cyan long-lasting phosphorescence (LLP) phosphor Ca3(PO4)2:Eu2+,Dy3+ was synthesized by a solid state reaction and its LLP properties were investigated for the first time. The Ca3(PO4)2:Eu2+,Dy3+ phosphor exhibits two emission bands centered at 480 nm and 573 nm, which are ascribed to the 4f65d1–4f7 electronic transition of Eu2+ ions and the transition from 4F9/2 to 6H13/2 of Dy3+ ions, respectively. After removal of the excitation source, the bright cyan LLP could be observed for 5 h by the naked eye. Besides, the yellow LLP component originated from the persistent energy transfer (PET) from Eu2+ ions to Dy3+ ions. The incorporation of Dy3+ ions creates more appropriate energy traps and evidently enhances the LLP behavior of Ca3(PO4)2:Eu2+ phosphor. A schematic model is constructed to convey trapping/detrapping processes and PET in the material.

 Please wait while we load your content...
Please wait while we load your content...