A practical and efficient route to heteraphanes: synthesis of structurally simplified analogues of ansamycins†
Abstract
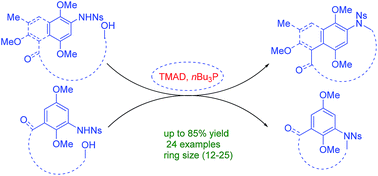
A highly efficient and versatile synthetic strategy has been developed for the synthesis of meta-, para-azabenzeno or azanaphthaleno phanes (II–IV) with varying ring sizes (12–25) through intramolecular Mitsunobu reaction from the corresponding Ns-arylamino alcohols. The reaction is compatible with olefin and ketone moieties in the backbone tether and has been applied in the synthesis of various simplified structural motifs of ansamycins.

 Please wait while we load your content...
Please wait while we load your content...