A phenomenon of degradation of methyl orange observed during the reaction of NH4TiOF3 nanotubes with the aqueous medium to produce TiO2 anatase nanoparticles
Abstract
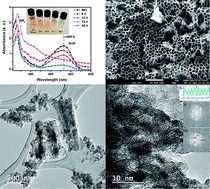
The phenomenon of decolourization of MO was observed when NH4TiOF3 nanotubes, in water, underwent a transformation toward anatase due to the effect of hydroxyl radicals. NH4TiOF3 nanotubes were synthesized using the electrochemical anodization method of titanium. They react within an aqueous solution producing nanoparticulated nanotubes formed of anatase crystals with a size of about 4–6 nm. A second reaction process was observed when the solution contained methyl orange (MO), which decomposed losing color, resulting in smaller molecules. The modified layer on the Ti anodized substrate was immersed in the aqueous solution in the absence of light. The degradation of MO was monitored by UV-Vis absorption. The highest degradation efficiency was obtained after 96 h. Sample analysis was conducted using liquid chromatography coupled with electrospray ionization ion-trap mass spectrometry. Six intermediate molecules were found during the degradation process of MO. Therefore, a degradation pathway of MO was proposed. The composition and surface structure of the compound were characterized. The analysis shows that NH4TiOF3 nanotubes change in morphology and crystal structure, showing nanotubes with nanoparticle walls and its evolution to anatase structure.

 Please wait while we load your content...
Please wait while we load your content...