Novel quinoxaline based chemosensors with selective dual mode of action: nucleophilic addition and host–guest type complex formation†
Abstract
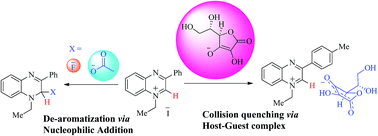
New quinoxalinium salts 1–5 have been exploited as chemosensors via naked eye, UV-Vis absorption, fluorescence quenching and 1H NMR experiments. New sensors 1–5 showed a dual mode, nucleophilic addition and a host–guest type complex towards anion (F−, AcO− and ascorbate) detection. Small anions (F−/AcO−) showed nucleophilic addition at the C2 position of the quinoxalinium cation, while larger anions (ascorbate), revealed the formation of a host–guest type complex due to the steric hindrance posed by the C3 of the phenyl ring. Nucleophilic addition of small anions (F−/AcO−) leads to the de-aromatization of the quinoxalinium cation. However in the case of the larger anion, ascorbate, the host–guest type complex formation induces changes in the absorption/fluorescence signals of the quinoxalinium moiety. This selective binding has been confirmed on the basis of the 1H NMR spectroscopic technique, whereupon nucleophilic addition of small anions (F−/AcO−) was confirmed by monitoring the characteristic proton NMR signals of Ha and the methylene protons (CH2), which were clearly shifted in the cases of fluoride and acetate ion addition confirming the de-aromatization and nucleophilic addition. Whereas no such peak shifting was observed in the case of ascorbate ion addition confirming the non-covalent addition of ascorbate. Theoretical insight into the selectivity and complexation behavior of the ascorbate ion with the quinoxaline moiety is gained through density functional theory (DFT) calculations. Moreover, the absorption properties of these complexes are modeled theoretically, and compared with the experimental data. In addition, the thermal decomposition of sensors (1 and 2) has been studied by the means of differential scanning calorimetry (DSC), thermogravimetry (TG), and differential thermogravimetry (DTG) to signify their utility at variable temperatures.


 Please wait while we load your content...
Please wait while we load your content...