Proximal environment controlling the reactivity between inorganic sulfide and heme-peptide model†
Abstract
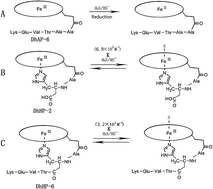
In order to gain a comprehension of the binding between inorganic sulfide and heme-containing proteins, we synthesized a series of deuterohemin-His-peptides (DhHPs) referred to as the active centers of cytochrome c as heme models to investigate the effects of the proximal environment on the reactivity when there is a lack of distal residues. DhHPs contain synthetic peptides with 2, 3, 4, 5, and 6 amino acids, respectively, whereas the imidazole side chain of His2 serves as the fifth ligand for all ferric centers. The coordination of sulfide to the ferric iron was confirmed by UV-Vis and EPR spectroscopy and the kinetic constants of the binding reaction were determined using a stopped-flow technique. Deuterohemin-AlaHisThrValGluLys (DhHP-6) exhibits an association rate constant kon = 2.34 × 104 M−1 s−1, which is 4-times faster than that found for deuterohemin-AlaHis (DhHP-2). The koff values of all the DhHPs are close and independent of the lengths and sequences of the peptides. It is hypothesized that the proximal environment plays an important role in the association processes, in which H-bond networks provided by proximal polar residues may facilitate the reaction. Moreover, the existence of Glu induces a substantial increase in the kon value of DhHP-5 and -6, indicating that the introduction of Glu5 enhances the reactivity of sulfide towards the ferric centers. We have also prepared a deuterohemin-β-Ala-β-Ala-Thr-Val-Glu-Lys (DhAP-6) derivative with Ala2 replacing His2. The results show that sulfide does not coordinate with the ferric center in DhAP-6, but reduces it to ferrous.

 Please wait while we load your content...
Please wait while we load your content...