What determines the volume transition temperature of UCST acrylamide–acrylonitrile hydrogels?
Abstract
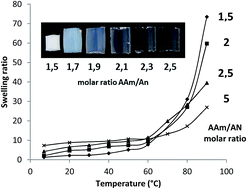
A series of covalently crosslinked hydrogels based on acrylamide and acrylonitrile were synthesized by means of free radical copolymerization using methylene bis(acrylamide) as crosslinker and ammonium persulfate–N,N,N′,N′-tetramethylethylenediamine (TEMED) as the redox-initiator system. These hydrogels possess a positive thermosensitivity and their volume expansion in aqueous solution upon temperature increase was monitored by measuring the swelling ratio between 7 and 90 °C. By changing a number of synthesis parameters such as molar ratio between acrylamide and acrylonitrile, crosslinker concentration, polymerization time and presence or absence of TEMED accelerator, the results point out that the apparent volume transition temperature of the hydrogels is mainly determined by the crosslinker concentration, i.e., the crosslinking density. Surprisingly, the composition-tunable UCST of uncrosslinked copolymers of acrylamide and acrylonitrile was not observed with the hydrogels. While the volume transition temperature appears to be insensitive to the composition, the actual change in swelling ratio going through UCST is larger for a hydrogel containing more acrylonitrile units, as a result of being more dehydrated below and more hydrated above UCST, respectively. Furthermore, without TEMED the polymerization time influences the swelling ratio and the transition temperature, while with TEMED it has little effect.

 Please wait while we load your content...
Please wait while we load your content...