Safer lithium metal battery based on advanced ionic liquid gel polymer nonflammable electrolytes†
Abstract
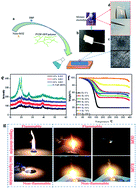
Uncontrolled parasitic side-reactions with metallic lithium and their hazardous behaviour hinders the development of advanced energy storage technologies based on organic solvents. Ionic Liquids (ILs) have very interesting and safer properties for the aim of realizing safer devices without hindering their electrochemical performance. Ionic liquid gel polymer electrolytes (ILGPEs) based on a micro-porous polymer membrane were designed to both improve the battery safety and maintain rapid migration channels for Li+, which possess a high ionic conductivity of 1.11 mS cm−1 at 25 °C with an electrochemical stability window up to 5 V versus Li/Li+ at room temperature. Moreover the ILGPEs increased compatibility with the lithium anode which suppressed the growth of lithium dendrites and lowered the SEI resistance. Furthermore, they also demonstrate excellent cycling stability with 91.1% capacity retention up to 100 cycles and a high columbic efficiency of 99% at 25 °C. With the addition of good thermal stability and non-volatile and non-flammable properties, all these features allow this novel gel polymer electrolyte to function as a high performance and highly safe lithium ionic conductor as well as a separator for lithium metal batteries.

 Please wait while we load your content...
Please wait while we load your content...