C–H⋯Br–C vs. C–Br⋯Br–C vs. C–Br⋯N bonding in molecular self-assembly of pyridine-containing dyes†
Abstract
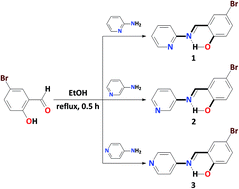
We have studied a series of closely related N-(5-bromosalicylidene)-x-aminopyridine compounds (x = 2, 1; 3, 2; 4, 3), obtained by condensation of 5-bromosalicylaldehyde with the corresponding aminopyridine. 1H NMR spectroscopy in solution revealed a single structure, at least in CDCl3. According to single crystal X-ray diffraction it was established that the crystal structures of 1–3 each are stabilized by a linear intramolecular hydrogen bond of the O–H⋯N type, formed between the hydroxyl hydrogen atom of the phenolic ring and the imine nitrogen atom. The dihalogen C–Br⋯Br–C and halogen C–Br⋯N(Py) interactions play a crucial role for the formation of supramolecular architectures in the structures of 2 and 3, respectively. The overall geometry of each molecule in the structures of 1 and 2 was found to be almost planar, while a significantly twisted structure was found for 3. Hirshfeld surface analysis showed that the structures of all compounds are mainly characterized by H⋯X contacts as well as by a remarkable contribution from C⋯C and C⋯N contacts which is clearly observed for 1 and 2. Diffuse reflectance spectra of 1–3 each exhibit a mixture of enol, cis-keto and trans-keto forms. A major contribution of the trans-keto form is found for 1 whereas a moderate fraction is detected for 2, and only traces of the trans-keto form were observed for 3. Contrary to expectations based on dihedral angle Φ considerations, 1 exhibits negative photochromism, although it was expected to be only thermochromic. Both 2 and 3 are not photochromic, whereas 3 was expected to be photochromic (Φ > 25°). Highly favoured C⋯C, C⋯N and O⋯Br intermolecular contacts as well as the absence of Br⋯Br dihalogen interactions in the structure of 1, are presumably mainly responsible for the photochromic behaviour. Furthermore, significantly impoverished H⋯C and H⋯N contacts further support the observed negative photochromism of 1.

 Please wait while we load your content...
Please wait while we load your content...