Hexavalent chromium induced tunable surface functionalization of graphite†
Abstract
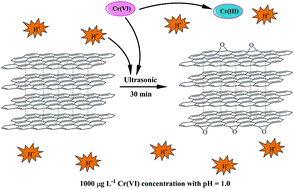
Hexavalent chromium (Cr(VI)) was chosen to serve as an oxidant to functionalize the surface of graphite. The effect of pH and concentration of Cr(VI) solution on the functionalization of graphite was systematically investigated by Raman spectroscopy, Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), and X-ray photoelectron spectroscopy (XPS). The results showed that the ether (C–O–C) functional groups were observed on the surface of graphite after being treated with pH = 1.0 solution with a Cr(VI) concentration of 1000 μg L−1 for 30 min. However, no obvious functional groups were observed on the graphite surface for the pH higher than 2.0 and the Cr(VI) concentrations higher than 2.0 mg L−1. The electrical conductivity of functionalized graphite by Cr(VI) was observed to be good, i.e., the same order of magnitude as that of the as-received graphite without a serious decrease. This work provides a new strategy to functionalize graphite for preparing multifunctional graphite nanocomposites.

 Please wait while we load your content...
Please wait while we load your content...