Enhanced adsorption capacity of polypyrrole/TiO2 composite modified by carboxylic acid with hydroxyl group†
Abstract
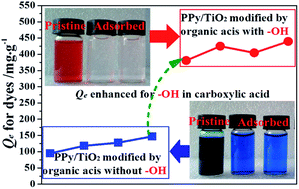
It was reported in our previous work that polypyrrole/TiO2 (PPy/TiO2) composite exhibited excellent adsorption ability for organic contaminants, and modification of the composite by different acids could greatly impact the adsorption performance. Herein, AC-PPy/TiO2, SU-PPy/TiO2, TA-PPy/TiO2 and CI-PPy/TiO2 composites were prepared in acetate (with only one carboxylic group), succinic (with two carboxylic groups), tartaric (with two hydroxyl and two carboxylic groups) and citric (with one hydroxyl and three carboxylic groups) acids, respectively. Acid Red G (ARG) as the typical anionic dye and Methylene Blue (MB) as the typical cationic dye were employed as the target removals to investigate the adsorption behavior of the as-prepared composites. It was found that the hydroxyl group in the employed carboxylic acids significantly affected the surface physicochemical properties and further the adsorption capacity of PPy/TiO2 composite. TA-PPy/TiO2 and CI-PPy/TiO2 prepared in the carboxylic acids with hydroxyl group displayed enhanced adsorption capacity for either ARG or MB, which was 3–4 times higher than that of AC-PPy/TiO2 and SU-PPy/TiO2 prepared in the carboxylic acids without hydroxyl group. Furthermore, all the composites could rapidly reach adsorption equilibrium within 30 min and could be reused at least 4 times without losing the adsorption capacity. The adsorption kinetic and thermodynamic data proved that the adsorption process of the employed dyes onto the composites mainly depended on the chemisorption.

 Please wait while we load your content...
Please wait while we load your content...