A unified approach to pyrrole-embedded aza-heterocyclic scaffolds based on the RCM/isomerization/cyclization cascade catalyzed by a Ru/B-H binary catalyst system†
Abstract
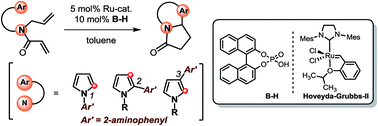
An easy and straightforward preparation of pyrrole-embedded aza-heterocyclic scaffolds employing a Ru/B-H binary catalyst system has been developed. The strategy generates a diverse array of privileged scaffolds from 2-aminophenyl group appended pyrroles that can be prepared by a two-step process from corresponding aminoaryl-substituted pyrroles. The technique of incorporating 2-aminoaromatic groups in the heterocycles and their subsequent ring-closing-metathesis (RCM) isomerization followed by subsequent Pictet–Spengler type reaction should also be applicable to other heterocycles for generating a library of multi-ring compounds in an efficient manner.

 Please wait while we load your content...
Please wait while we load your content...