Preparation and electrochemical properties of double-shell LiNi0.5Mn1.5O4 hollow microspheres as cathode materials for Li-ion batteries†
Abstract
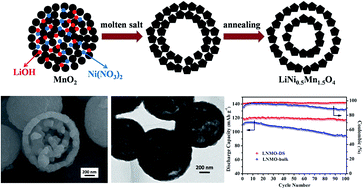
Double-shell LiNi0.5Mn1.5O4 (LNMO-DS) hollow microspheres have been synthesized via a facile molten salt and annealing method. This method is a heating and cooling process with programmed control, which can promote the formation of a double-shell hollow microspherical structure and suppress rock-salt impurity phase. The LNMO-DS material with an average size of about 1 μm has outer and inner shells with thicknesses of all about 100 nm, which is confirmed by transmission electron microscopy (TEM). The double shell structures allow easy penetration of the electrolyte into the whole microspheres and buffer the large volume change of the electrode materials during Li ion intercalation/deintercalation processes. When applied as cathode materials for Li ion batteries, LNMO-DS exhibit high reversible capacity, excellent cycling and rate performances. The capacities remain at about 98.3% after 100 cycles (116.7 mA h g−1 at 0.5C). Furthermore, the favorable electrochemical performances of LNMO-DS are suitable for them to be used as the positive electrode in full cells.

 Please wait while we load your content...
Please wait while we load your content...