Exploring coumarin potentialities: development of new enzymatic inhibitors based on the 6-methyl-3-carboxamidocoumarin scaffold†
Abstract
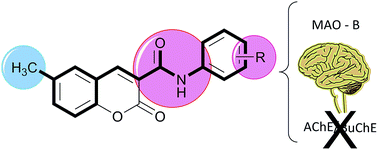
Novel 6-methyl-3-carboxamidocoumarins (compounds 4–15) were synthesized by an effective three step synthetic strategy and screened towards MAO, AChE and BuChE enzymes. In general, the compounds act as selective MAO-B inhibitors. Compound 11 is highlighted as a potent (IC50 hMAO-B = 4.66 nM), reversible and non-competitive MAO-B inhibitor.

 Please wait while we load your content...
Please wait while we load your content...