Unraveling the dynamic nature of protein–graphene oxide interactions†
Abstract
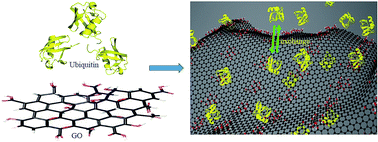
In recent years, Graphene Oxide (GO) and its derivatives have attracted significant attention owing to their unique physicochemical, optical and conductive properties and have been used in a diverse range of applications. One of the properties of GO, which is important for its applications in biological systems, is the nature of its interactions with biomolecules such as proteins. We present here the dynamic aspects of the interaction of GO with human ubiquitin (8.6 kDa) unraveled using nuclear magnetic resonance (NMR) spectroscopy. This study, for the first time, reveals an interaction involving fast and reversible association–dissociation of the protein molecules from the surface of the GO sheet. The conformation of the protein is not affected due to the interactions. The interactions were found to be electrostatic in nature and are attributed to the polar functional groups present on the protein and GO sheets, which was verified by titration of GO with ubiquitin at different pH. Taken together, the study provides new insights into protein–GO interactions, and the NMR methods described will be of utility for probing such interactions in general while designing new chemical and biological applications involving functionalized graphene oxide.


 Please wait while we load your content...
Please wait while we load your content...