Immobilization of a thiol-functionalized ionic liquid onto HKUST-1 through thiol compounds as the chemical bridge
Abstract
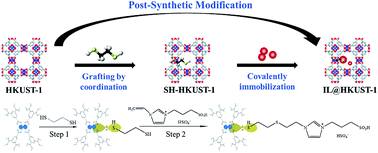
A novel heterogeneous catalyst [HVIm-(CH2)3SO3H]HSO4@HKUST-1 (IL@HKUST-1), with both Lewis and Brønsted acid sites, was developed for the esterification of oleic acid with short-chain alcohols. HKUST-1 was chemically modified with ethanedithiol, and the vinyl-containing ionic liquid was then grafted onto the carrier through thiol groups. The catalyst IL@HKUST-1 was characterized by XRD, N2 adsorption–desorption, FT-IR, SEM, TG, elemental analysis, and ICP. The results proved that HKUST-1 had typical microporous structure, and the thiol groups were incorporated into the channels of the carrier. Through the reaction of vinyl and thiol, the ionic liquid was successfully immobilized onto SH-HKUST-1 by chemical covalent bonds. The catalyst was applied in the esterification of oleic acid with ethanol, and the optimal conditions were determined as follows: molar ratio of ethanol to oleic acid 12 : 1, catalyst amount 15 wt% (based on oleic acid), reaction time 4 h, and reaction temperature 90 °C. Under the conditions, the conversion of oleic acid was 92.1%. After 5 times of recycling, there was no significant decrease in conversion, showing a certain stability and good reusability of the catalyst. The catalyst also exhibited high catalytic activity in esterification of oleic acid with other short-chain alcohols.

 Please wait while we load your content...
Please wait while we load your content...