A straightforward sequence to alkyl 1H-pyrrole-2,5-dicarboxylates starting from acylhydrazono esters and alkyl 2-aroyl-1-chlorocyclopropanecarboxylates†
Abstract
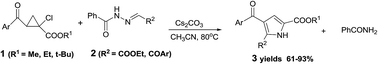
A highly regioselective domino reaction was developed between alkyl 2-aroyl-1-chlorocyclopropanecarboxylates 1 and acylhydrazono esters 2 in the presence of Cs2CO3 under mild basic conditions, which directly afforded 2,3,5-trifunctionalized pyrroles in good to excellent yields with the extrusion of benzamide. A plausible pathway was proposed involving 1,2-elimination, regioselective nucleophilic addition, the intramolecular Mannich reaction, and the removal of benzamide to rationalize the formation of the aromatic pyrrole system.

 Please wait while we load your content...
Please wait while we load your content...