Hemocompatible glutaminase free l-asparaginase from marine Bacillus tequilensis PV9W with anticancer potential modulating p53 expression†
Abstract
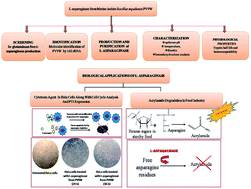
Bacillus tequilensis PV9W, a marine bacterial isolate obtained from Gulf of Mannar, Rameswaram, India, produced glutaminase free L-asparaginase which was purified to homogeneity with a significant increase (13 fold) in specific activity. The apparent Km (0.045 ± 0.013 mM) and Vmax (7.465 ± 0.372 μmol ml−1 min−1) values of this purified L-asparaginase was identified and it was found that the maximum activity of the L-asparaginase was at a pH 8.5 and a temperature of 35 °C. The enzyme is a mixed α/β protein and the influence of different effectors were documented. The purified L-asparaginase had effective acrylamide degradation activity (6 IU per ml) and cytotoxic activity against HeLa cell lines with an IC50 of 0.036 ± 0.009 IU per ml. Furthermore, the purified enzyme showed p53 dependent G2 arrest in HeLa cells analyzed by FACS and was hemocompatible. Thus, this study highlights the marine isolate PV9W as a potential source for glutaminase free L-asparaginase with industrial as well as pharmaceutical applications. This study also paves a way for a possible therapeutic drug with the least amount of side effects.

 Please wait while we load your content...
Please wait while we load your content...