π-Bonding-dominated energy gaps in graphene oxide
Abstract
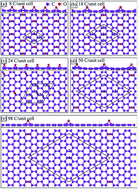
The chemical bonding in graphene oxide with oxygen concentrations from 50% to 1% is investigated using first-principle calculations. The energy gaps are mainly determined by the competition of the orbital hybridizations in the C–C, O–O, and C–O bonds. They are very sensitive to changes in the oxygen concentration and distribution. Five types of π-bonding exist on moving from full to vanishing adsorption, namely complete termination, partial suppression, 1D bonding, deformed planar bonding, and the well-behaved type. They can account for the finite and gapless characteristics, corresponding to O-concentrations of >25% and <3%, respectively. The feature-rich chemical bonding dominates the band structures and density of states, leading to diverse electronic properties.

 Please wait while we load your content...
Please wait while we load your content...