Novel thiosemicarbazide–oxadiazole hybrids as unprecedented inhibitors of yeast α-glucosidase and in silico binding analysis†
Abstract
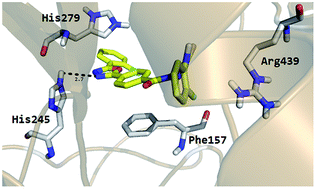
Compounds 1–18, new oxadiazole–thiosemicarbazide hybrids, were synthesized using a five-step reaction sequence in excellent yields. All the synthesized analogs exhibited exceptional α-glucosidase inhibitory potentials in the range of 0.4–38.1 μM. Among the current series, it was observed that the fluoro-substituted analogues were exceptionally potent inhibitors of α-glucosidase. The study provides a proof of concept that inductively strong electron withdrawing groups result in enhanced inhibitory potentials. The binding interactions of these compounds were analyzed in silico for possible prediction and identification of the improved inhibitory potential.

 Please wait while we load your content...
Please wait while we load your content...