Magnetic structure of (C5H12N)CuBr3: origin of the uniform Heisenberg chain behavior and the magnetic anisotropy of the Cu2+ (S = 1/2) ions†
Abstract
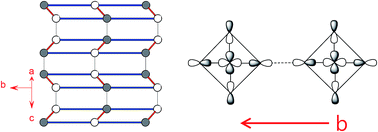
The magnetic properties and electric polarization of the organic/inorganic hybrid system (C5H12N)CuBr3 (C5H12N = piperidinium) were examined on the basis of density functional theory calculations. The spin exchanges of (C5H12N)CuBr3 evaluated by energy-mapping analysis show that its uniform Heisenberg antiferromagnetic chain behavior is not caused by the CuBr3 chains made up of edge-sharing CuBr5 square pyramids, but by the two-leg spin ladders resulting from interchain interactions. The magnetic anisotropy of the Cu2+ ions in (C5H12N)CuBr3 originates largely from the Br− ligands rather than the Cu2+ ions. The electric polarization of (C5H12N)CuBr3 arises from the absence of inversion symmetry in the crystal structure, and is weakly affected by the magnetic structure.

 Please wait while we load your content...
Please wait while we load your content...