Excellent lithium ion storage property of porous MnCo2O4 nanorods†
Abstract
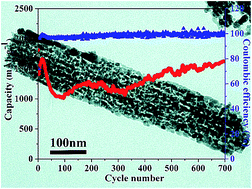
In this report, we present a facile method for the synthesis of porous MnCo2O4 nanorods. When the as-synthesized MnCo2O4 nanorods are applied as the anode material for lithium-ion batteries, they exhibit excellent electrochemical performance owing to their porous nature. Because of the delicate balance between structural stability and specific surface area, the porous MnCo2O4 nanorods show high discharge capacity and excellent cycling stability. The initial discharge capacity is 1845 mA h g−1 at a current density of 0.4 A g−1. Even being cycled at a current density of 30 A g−1, the discharge capacity is still about 533 mA h g−1. The effects of binders on the electrochemical performance of the electrode have also been investigated, showing that the CMC/SBR binder is more suitable than PVDF for the as-synthesized porous MnCo2O4 nanorods. The influences of the calcination temperature also have been systematically investigated, which suggest the electrochemical performance is determined by both the structural stability and surface area of the samples.

 Please wait while we load your content...
Please wait while we load your content...