Reactivity of norbornene esters in ring-opening metathesis polymerization initiated by a N-chelating Hoveyda II type catalyst
Abstract
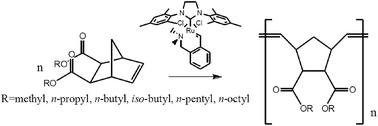
The reactivity and activation parameters for the ring-opening metathesis polymerization of eight norbornene esters in the presence of a N-chelating Hoveyda–Grubbs II type catalyst were determined using in situ 1H-NMR. The ester molecules differ in the structure of substituent and the location of ester groups. Kinetic studies have shown that effective polymerization constants and activation parameters highly depend on the monomer structures. It was demonstrated that the elongation of the aliphatic chain does not significantly affect the reactivity of the ester, but has a high impact on the activation parameters of the reaction. Branching of the aliphatic substituent has a significant influence on the reactivity and activation parameters. Furthermore, the orientation of the ester substituents in the norbornene ring substantially affects the activation parameters.

 Please wait while we load your content...
Please wait while we load your content...