DFT study on the CuBr-catalyzed synthesis of highly substituted furans: effects of solvent DMF, substrate MeOH, trace H2O and the metallic valence state of Cu†
Abstract
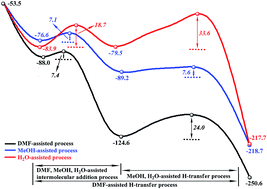
The CuBr-catalyzed 2-(1-alkynyl)-2-alken-1-ones are selected as a research system to explore the effects of solvent DMF, substrate MeOH, trace H2O and the valence state of Cu on the synthesis of highly substituted furans using the BHandHLYP density functional. Our calculations suggest that DMF, MeOH and H2O can be used as hydrogen-bond acceptors to accelerate intermolecular nucleophilic addition. More importantly, they play the role of proton shuttles to assist H migration significantly by reducing the free energy barrier of the H-transfer process. Due to the participation of DMF, MeOH or H2O, the rate-determining step is changed from the H-transfer process (96.0 kJ mol−1) to the intramolecular cyclization step (57.6 kJ mol−1). In addition, calculated results also show that the yield can be further improved when CuBr is replaced by CuBr2. In short, the present study can provide insight into the metal-catalyzed reactions involving H-transfer processes and can act as a guideline for the design of new catalysts for metal-catalyzed reaction applications.

 Please wait while we load your content...
Please wait while we load your content...