Peculiarities of 2-amino-3-R-4-aryl-4H-pyranes multicomponent synthesis derived from 1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide†
Abstract
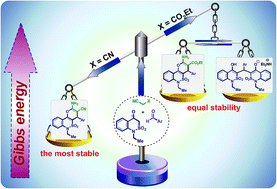
The new 2-amino-3-R-4-aryl-6-ethyl-4,6-dihydropyrano[3,2-c][2,1]benzothiazine 5,5-dioxides were synthesized via three-component interaction of 1H-2,1-benzothiazin-4(3H)-one 2,2-dioxide with arylcarbaldehydes and active methylene nitriles. Depending on the nature of an active methylene nitrile and an arylcarbaldehyde this interaction can lead either to the target 2-amino-4H-pyrans or to the stable triethylammonium salts of bis(1H-2,1-benzothiazin-4(3H)-one 2,2-dioxides) (bis-adducts). The latter is a completely new product of such interaction. The structure of the bis-adduct was confirmed by single crystal X-ray diffraction. Actually, the formation of stable triethylammonium salts (as the process competitive with the 2-amino-4H-pyrans formation) appeared to be reversible and their interaction with active methylene nitriles led to the formation of 2-amino-4H-pyrans. The extended and adjusted mechanism of the three-component interaction, that includes the bis-adducts formation stage, was proposed. Taking into account the peculiarities of the mechanism, we were capable to control the reaction selectivity.

 Please wait while we load your content...
Please wait while we load your content...