Pyridyl vs. bipyridyl anchoring groups of porphyrin sensitizers for dye sensitized solar cells†
Abstract
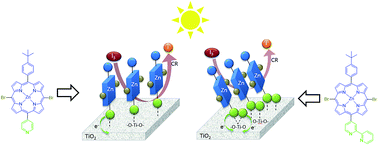
The synthesis of two porphyrins with donor–π–acceptor (D–π–A) molecular architecture is described, namely Znpor-py (3a) and Znpor-bpy (3b), which consist of a 4-tert-butyl-phenyl group as donor and either a pyridine or a bipyridine group as acceptor at opposite positions of the porphyrin macrocycle. Photophysical and electrochemical properties of the two compounds, as well as theoretical DFT calculation results suggest that the two porphyrins have the potential to act as sensitizers in dye-sensitized solar cells (DSSCs). Both dyes contain N(pyridyl) atoms able to act as anchors onto the acid sites of TiO2. A Znpor-py (3a) sensitized solar cell was found to exhibit power conversion efficiency (PCE) of 3.57%, while the corresponding Znpor-bpy (3b)-functionalized solar cell showed a higher PCE of 5.08%. The enhanced short circuit current Jsc and open circuit voltage Voc parameters are the main factors responsible for the improved photovoltaic performance of the latter solar cell. These are attributed to its faster charge injection into the TiO2 photoanode and its reduced charge recombination at the electrode/electrolyte interface, which result from the stronger binding and coordination geometry of the bipyridine anchoring group on TiO2. Electrochemical impedance spectra (EIS) of the two solar cells further support these assumptions, revealing a higher charge recombination resistance Rrec and a longer electron lifetime τe for the Znpor-bpy (3b) sensitized solar cell.

 Please wait while we load your content...
Please wait while we load your content...