Pyrazine-based donor tectons: synthesis, self-assembly and characterization†
Abstract
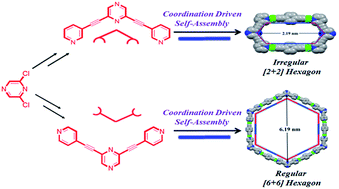
Two new supramolecular building blocks derived from pyrazine are introduced. These molecules, having pendant pyridine units covalently linked to central pyrazine ring, are structurally rigid with pre-defined bite angles. Therefore they can act as donor tectons in design of supramolecular hexagons using coordination driven self-assembly protocol. Multinuclear NMR (including 1H DOSY) and mass spectrometry have been utilized to confirm the purity and stoichiometry of these self assembled hexagonal ensembles. PM6 molecular modeling studies corroborate their hexagonal shape and nanoscalar dimensions.

 Please wait while we load your content...
Please wait while we load your content...