Radiosynthesis and characterisation of a potent and selective GPR139 agonist radioligand†
Abstract
Compound 1 is a selective and potent agonist of the G protein-coupled receptor GPR139 (EC50 = 39 nM). In this study, we describe the synthesis, radiolabelling and in vitro evaluation of [3H]-1 for the characterisation of GPR139 and its spatial expression in the brain using autoradiography. Two different synthesis routes for the radiolabelling of 1 based on a reductive debromination strategy were investigated using deuterium (D2, g). The route based on reductive debromination of the bromonaphthyl precursor 5 proved superior over arylbromide 4 and was employed for the radiolabelling experiments. Reductive debromination of precursor 5 was accomplished using 3H2, Pd/C and triethylamine in DMF at ambient temperature to give target molecule [3H]-1 with a specific activity of 19.3 Ci mmol−1 and a radiochemical purity of ≥95%. By application of autoradiography and binding studies, it was not possible to discriminate [3H]-1 binding to wildtype mice brains from GPR139 knockout mice brains and total binding from non-specific binding in CHO-k1 cells stably expressing human GPR139 receptor. Based on these experiments we conclude that [3H]-1 is not a suitable radioligand for the characterisation of GPR139.
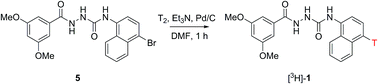


 Please wait while we load your content...
Please wait while we load your content...