The facile and stereoselective synthesis of pyrrolidine β-amino acids via copper(i)-catalyzed asymmetric 1,3-dipolar cycloaddition†
Abstract
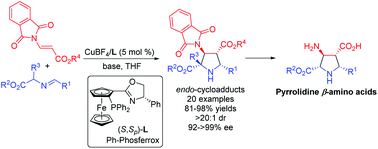
A highly efficient copper(I)-catalyzed asymmetric 1,3-dipolar cycloaddition of azomethine ylides with β-phthaliminoacrylate esters was developed under mild conditions, providing the desired pyrrolidine β-amino acid derivatives in high yields (up to 98%) with excellent diastereo- and enantioselectivities (dr >20 : 1, ee up to >99%). Subsequent deprotection and hydrogenolysis of the corresponding cycloadducts could deliver highly functionalized and biologically important free pyrrolidine β-amino acids in a straightforward and efficient pathway.
- This article is part of the themed collection: HOT articles in Organic Chemistry Frontiers in 2016

 Please wait while we load your content...
Please wait while we load your content...