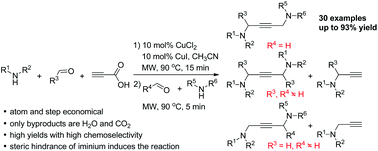
Controlling chemoselectivity in copper-catalyzed decarboxylative A3/A3 cross-couplings: direct formation of unsymmetrical 1,4-diamino-2-butynes†
Abstract
A direct cross-coupling of propiolic acid with two kinds of in situ formed iminiums has been achieved via the CuI/CuCl2-catalyzed decarboxylative A3/A3 domino process. This novel protocol to synthesise unsymmetrical 1,4-diamino-2-butynes is operationally simple in an atom- and step-economical manner, and provides products in moderate to excellent yields with high chemoselectivity.

 Please wait while we load your content...
Please wait while we load your content...