Iodine-mediated regioselective 5-endo-dig electrophilic cyclization reaction of selenoenynes: synthesis of selenophene derivatives†
Abstract
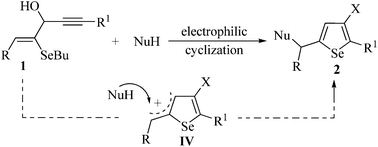
Selenoenynes underwent electrophilic cyclization reactions with iodine in the presence of an appropriate nucleophile to give 3-iodo-selenophenes and 3-organoselenyl-selenophenes. The selection of the appropriate reaction conditions allowed the cyclization process, which involves the initial formation of an iodonium intermediate, followed by a regioselective 5-endo-dig intramolecular selenium nucleophilic attack to the carbon–carbon triple bond. The key step for the preparation of the desired selenophenes was the trap of an allylic cation intermediate by N- and O-nucleophiles. The generality and limitations of the reactions with regard to each of the substituents at the starting materials were evaluated and addressed. The resulting 3-iodo-selenophenes were further functionalized by Sonogashira and Suzuki cross-coupling reactions.


 Please wait while we load your content...
Please wait while we load your content...