Nickel-catalyzed alkyl-zincation and carboxylation of diynes†
Abstract
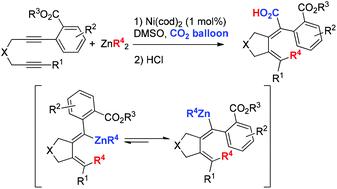
A nickel-catalyzed three-component carbo-carboxylation of aryl-substituted diynes with ZnR2 and CO2 is described. With an ortho-ester functionality in the aryl group as the directing group, the usually observed hydrocarboxylation is completely suppressed with an unexpected C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond isomerization. Based on the X-ray diffraction analysis and mechanistic study, it is believed that the reaction started with the oxidative addition with an aryl-substituted C–C triple bond and transmetalation with dialkyl zinc, followed by syn-carbonickelation with an alkyl-substituted C–C triple bond. Subsequent reductive elimination afforded the corresponding sp2-carbon zinc intermediate. Finally, the dynamic isomerization of a zinc-linked C
C bond isomerization. Based on the X-ray diffraction analysis and mechanistic study, it is believed that the reaction started with the oxidative addition with an aryl-substituted C–C triple bond and transmetalation with dialkyl zinc, followed by syn-carbonickelation with an alkyl-substituted C–C triple bond. Subsequent reductive elimination afforded the corresponding sp2-carbon zinc intermediate. Finally, the dynamic isomerization of a zinc-linked C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond and the subsequent exclusive reaction of an isomerized sp2-carbon zinc intermediate with CO2 afforded the final carboxylic acids with a high stereoselectivity.
C bond and the subsequent exclusive reaction of an isomerized sp2-carbon zinc intermediate with CO2 afforded the final carboxylic acids with a high stereoselectivity.

 Please wait while we load your content...
Please wait while we load your content...